There are many different types of HIV medication, and new HIV medicines are being developed all the time. The current medication is effective and safe. However, some people suffer from side effects, so switching to a different medication may be a good idea.
In brief:
- They can be divided into five classes.
- HIV can become resistant if you don't take your medication consistently.
- Always report side effects both to your doctor and on www.lareb.nl, you are then helping to improve HIV care.
- Generic drugs are copies of a drug after its patent has expired.
- HIV medication may interact with other medication, supplements or herbs.
- HIV medication can also be used to prevent HIV.
Plenty of choice
There are various classes and combinations of HIV medication available. So there is a medication to suit everyone. Sometimes it takes a while to find out which medication is the best fit, and a switch may be necessary. Talk to your doctor or nurse if you want to switch, so that you can find the right medication for you. HIV medication keeps the virus suppressed, so you can't transmit HIV.
Types of HIV medication in the Netherlands
There are several names for each HIV medication. A drug has a chemical name that is written with a lowercase letter, and falls into a class. Besides the name of the class and the name of the chemical, the drug also has a brand name that is written with a capital letter: e.g. Stocrin. The brand name can vary from country to country, Stocrin is called Sustiva in some countries, for example. The active ingredient in Stocrin is efavirenz, in the class non-nucleoside reverse transcriptase inhibitors (NNRTIs).
When the drug is still being researched, it often only has a code name. The code name gets changed to a chemical name during the research, later on it gets a brand name. Medication can’t be marketed in the Netherlands until it has been licensed by the CBG (Medicines Evaluation Board). Read more about research into new HIV medication here. New drugs also appear in updates on de medische blog (in Dutch).
These HIV medications are available in the Netherlands
- Single-pill regimens (one pill a day)
- Atripla* (emtricitabine + efavirenz + tenofovir disoproxil), licensed since 2007
- Eviplera (emtricitabine + rilpivirine + tenofovir disoproxil), licensed since 2011
- Stribild (elvitegravir + emtricitabine + tenofovir disoproxil + booster cobicistat), licensed since 2013
- Triumeq (abacavir + dolutegravir + lamivudine), licensed since 2014
- Genvoya (elvitegravir + emtricitabine + tenofovir alafenamide + booster cobicistat), licensed since 2015
- Odefsey (emtricitabine + rilpivirine + tenofovir alafenamide), licensed since 2016
- Symtuza (darunavir + emtricitabine + tenofovir alafenamide + booster cobicistat), licensed since 2017
- Juluca (dolutegravir + rilpivirine), licensed since 2018
- Biktarvy (bictegravir + emtricitabine + tenofovir alafenamide), licensed since 2018
- Delstrigo (doravirine + lamivudine + tenofovir disoproxil), licensed since 2018
- Dovato (dolutegravir + lamivudine), licensed since 2019
- Long-acting injections (combined, every 8 weeks)
- Vocabria (cabotegravir-injection), licensed since 2020
- Rekambys (rilpivirine-injection), licensed since 2020
- Fixed-dose combinations (two NRTIs, to be used as backbone)
- Combivir* (lamivudine + zidovudine), licensed since 1998
- Kivexa* (abacavir + lamivudine), vergunning sinds 2004
- Truvada* (emtricitabine + tenofovir disoproxil), licensed since 2005
- Descovy (emtricitabine + tenofovir alafenamide), licensed since 2016
- Nucleoside/nucleotide reverse transcriptaseremmer (NRTI)
- Epivir* (lamivudine), licensed since 1996
- Ziagen* (abacavir), licensed since 1999
- Viread* (tenofovir disoproxil), licensed since 2001
- Emtriva (emtricitabine), licensed since 2003
- Vemlidy (tenofovir alafenamide), licensed since 2017
- Intregrase inhibitor (INI)
- Isentress (raltegravir), licensed since 2007
- Tivicay (dolutegravir), licensed since 2014
- Vocabria (cabotegravir), licensed since 2020
- Non-nucleoside/nucleotide reverse transcriptaseremmer (NNRTI)
- Viramune* (nevirapine), licensed since 1998
- Stocrin* (efavirenz), licensed since 1999
- Intelence (etravirine), licensed since 2008
- Edurant (rilpivirine), licensed since 2011
- Pifeltro (doravirine), licensed since 2018
- Protease inhibitor (PI)
- Kaletra* (lopinavir + booster ritonavir), licensed since 2001
- Reyataz* (atazanavir), licensed since 2004, no booster
- Prezista* (darunavir), licensed since 2007, no booster
- Rezolsta (darunavir + booster cobicistat), licensed since 2014
- Evotaz (atazanavir + booster cobicistat ), licensed since 2015
- Entry inhibitors
- Fuzeon (enfuvirtide), licensed since 2003
- Celsentri (maraviroc), licensed since 2007
- Trogarzo (ibalizumab), licensed since 2019
- Rukobia (fostemsavir), vergunning sinds 2021
- Booster
- Norvir* (ritonavir), licensed since 1996
- Tybost (cobicistat), licensed since 2013
* generic version available
One pill a day
A number of commonly used combinations of antiretroviral drugs are now available in a so-called combination therapy: several active ingredients brought together in one pill. In 2006, a pill combining three active ingredients, constituting the entire treatment, came on the market for the first time: the single-tablet regimen (STR). Since 2019, ten of these single-tablet regimens have been available, meaning that a large proportion of people living with HIV only need to take one pill a day.
Generic HIV medication
The pills above with an * have generic versions available. A generic medicine is a copy of a branded medicine. A generic medicine contains the same active ingredients, in the same amounts, making it as effective as the same branded medicine. It does look different. A generic medicine is often much cheaper than the branded medicine, and can be produced when the patent of the branded medicine has expired. Very occasionally, the side effects can differ. This may, for instance, be because a different filler is used.
What does HIV medication cost?
HIV medication is relatively expensive because it almost always involves a combination of at least three drugs. Treatment with HIV medication costs on average just under €1,000 a month. Because HIV medication was developed relatively recently, many of them still have patents. A small number of drugs are now on the market in generic (non-patented) versions, which has lowered the price of these medicines. The health insurers in the Netherlands reimburse the costs of all officially registered combination therapies.
How does HIV medication work?
HIV medication suppresses the HIV virus in a number of steps. The most commonly used combinations suppress the virus at two different stages. This is usually by inhibiting the enzyme (protein) reverse transcriptase, plus an additional step. This might be by inhibiting the enzymes protease and integrase. Inhibiting these enzymes prevents the formation of new HIV RNA, by preventing the slicing of viral proteins. Other HIV medication prevents HIV from entering cells, preventing the infection of new cells.
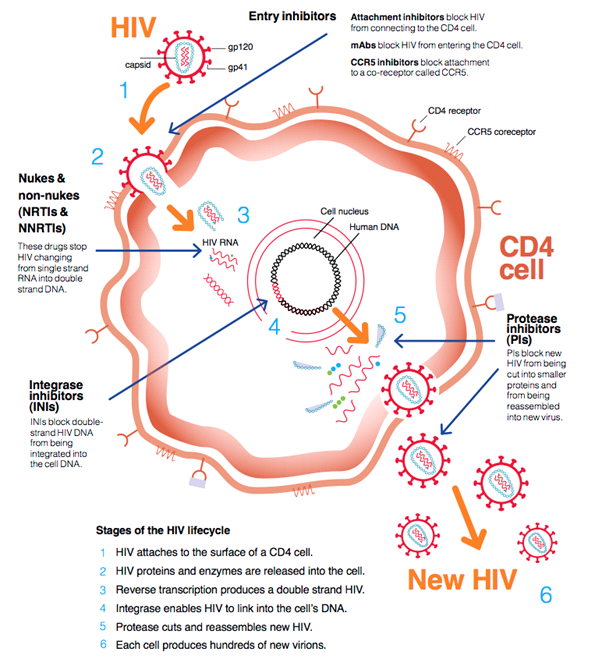
Bron: HIV i-Base
NRTI
NRTI stands for nucleoside/nucleotide reverse transcriptase inhibitor. The reverse transcriptase enzyme is unique to retroviruses like HIV. Once the virus has spread genetic material into the cell, reverse transcriptase converts the viral RNA to DNA. The DNA is used to make new virus particles. Reverse transcriptase inhibitors prevent new virus particles from being made. Common practice is to establish a combination therapy of two different nucleoside reverse transcriptase inhibitors (NRTI) supplemented by a non-nucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), or integrase inhibitor (INI).
Viral load = efficacy
You determine the efficacy of HIV medication by measuring the viral load. The viral load represents the number of HIV particles per millilitre of blood. Within a few months of starting the combination therapy, the viral load should be below the measurable limit (= 50 copies/ml), and should stay under this limit. This is called an undetectable viral load.
If the viral load becomes detectable at a later stage, this indicates that resistance to the drug being used has developed, so it doesn't work properly anymore. The medicine is clearly no longer sufficiently effective. In that case one should consider taking a different combination, making resistance not an issue.
Checking the number of CD4 cells is becoming less important, and the number of CD4 cells is no longer measured twice a year in everyone. This used to be very important because the viral load couldn’t be measured then. Now we know that the CD4 cell count is not a good indicator of someone's overall health. There are people with low CD4 cell counts, who are perfectly healthy. Measuring the viral load tells you more about the status of the HIV infection.
Side effects and switching
Sometimes it's a good idea to switch to another HIV medication; for instance, if you are experiencing side effects. Click here for more information on switching HIV medication. Click here for more about side effects.
Resistance and treatment adherence
A very small percentage of people with HIV have a virus that is resistant to one or more types of HIV medication. This can be caused by someone not taking their medication consistently, in which case they are not adhering to their treatment regime. Read more here about treatment adherence. Very occasionally someone gets infected with a virus that is already resistant. If you have contracted a resistant virus, your doctor will discuss this with you.
Blood count
You can prevent the development of a resistant virus by adhering to your treatment regime. With treatment adherence, the blood count (the amount of HIV medication in your blood) stays consistently high. That's why you should take the HIV medication on time and in the prescribed doses. If the blood count is too low because someone is not taking his or her HIV medication properly, HIV can adapt to the medicine and the virus can become resistant. Blood count determination can reveal more. The blood count is a measure of how much HIV medicine is in the blood. The blood count should not fall below or exceed a certain value. A low blood count could mean that the medicine is not sufficiently suppressing your HIV. A high blood count could increase the risk of side effects.
Interactions
HIV medication can have interactions with other medicines. This can influence the effectiveness and side effects of the medication.
Interaction means, that one drug increases or decreases the effectiveness of the other drug. If HIV medication A decreases the amount of drug B in the blood, then drug B will be less effective. If A actually increases the amount of B, then the side effects associated with B will become more severe.
There can be interactions between:
- One HIV medication and another
- HIV medication and other medication, including non-prescription medicines
- HIV medication and alternative medicines
- HIV medication and the birth control pill
- HIV medication and drugs, including ecstasy, speed, amphetamines, methadone and opiates
The interaction can go in two directions:
- The HIV medication influences the effectiveness or side effects of the other drug.
- The other drug influences the effectiveness or the side effects of the HIV medication.
A few examples of interactions:
- Ritonavir massively enhances the effect of ecstasy (XTC); a few people have died from an ecstasy overdose, when they had only taken a regular dose of ecstasy.
- A number of HIV medications reduce the effect of methadone.
- St John's wort reduces the effectiveness of many HIV medications.
- Many HIV medications increase the side effects of a number of sleeping pills and anti-anxiety drugs.
- There doesn't seem to be any interaction between cannabis and HIV medication.
- There is an interaction between alcohol and abacavir. Alcohol raises the effect of abacavir. This interaction is not serious with moderate alcohol consumption.
To avoid unwanted interactions, it is important to tell the HIV practitioner what medication you are taking in addition to the HIV medication. If you use drugs like ecstasy, opiates or methadone, it is important your HIV practitioner knows this. If you start taking a new medicine, you can ask your pharmacist or prescribing physician to check for interactions with your HIV medication.
Preventive effect of HIV medication
One aspect of HIV medication that is receiving increasing attention is the concept of preventive treatment. Ever since the discovery of effective HIV medication, researchers have suspected that suppressing the virus when treating people with HIV would also greatly reduce the extent to which they could transmit the virus. It was also discovered that HIV medication could be used to prevent someone from becoming infected.
U=U / TasP
TasP stands for Treatment as Prevention. In 2008, Swiss experts suggested that when people with HIV have been successfully treated – and they have a partner who doesn’t have HIV – they do not have to use a condom, subject to certain conditions. Since then, additional research in this field has been conducted, including the HPTN-052 study, the PARTNER1 study (2016 - in English), the Opposites Attract (2018 - in English) and the PARTNER2 study (2019 - in English).
We now know that people with HIV who have a suppressed virus cannot transmit HIV by having sex. We call this: undetectable = untransmittable. So treating people with HIV prevents transmission. And that is not only in the interest of the person with HIV, but also an important tool in fighting the epidemic and the stigma. That is why the Hiv Vereniging (Dutch Association of People with HIV), following the example of U=U, set up the campagne n=n (in Dutch). The message is the same – if the virus is not detectable you can't transmit HIV.
Post-treatment control
When you get HIV, the virus multiplies rapidly in the body and the viral reservoir gets larger and larger. Several studies have shown that the sooner a person starts taking HIV medication, the smaller the reservoir remains. Read more here about the viral reservoir.
After temporary treatment with HIV medicine, some people with a small viral reservoir are able to suppress the virus for a longer period, without taking any more HIV medication. This is called post-treatment control. Probably people treated soon after being infected are more likely to receive post-treatment control than people who are treated later. Eventually the viral load always goes up again in post-treatment control people, but this varies from person to person and you never really know when this will happen.









